When you’re pregnant, every pill you take feels like a decision that could change your baby’s future. You’ve heard the warnings: generic medications might not be safe. Maybe your aunt swore off them after a bad experience. Or your pharmacist offered a cheaper version and you froze, unsure if it was the right choice. The truth? Most generic drugs are just as safe during pregnancy as their brand-name cousins. But the confusion? That’s real.
What Exactly Is a Generic Drug?
A generic drug isn’t a knockoff. It’s not a copycat. It’s the same medicine, made to the exact same standard. The active ingredient-the part that actually works-is identical. If your doctor prescribes levothyroxine for hypothyroidism, whether you get Synthroid or a generic version, your body gets the same hormone, at the same strength, in the same way. The U.S. Food and Drug Administration (FDA) requires this. Generic manufacturers must prove their product behaves the same in your bloodstream as the brand-name version. That’s called bioequivalence. For most drugs, that means the amount absorbed must fall between 80% and 125% of the brand. For critical drugs like levothyroxine, the range is tighter: 90% to 112%. Why? Because even small changes in thyroid hormone levels during pregnancy can raise miscarriage risk by 61% and preterm birth risk by 39%.Why Do People Worry About Generics in Pregnancy?
It’s not irrational. Pregnancy changes your body. Your stomach empties slower. Your blood volume increases. Your kidneys filter faster. These shifts can affect how drugs move through you. So if two pills have the same active ingredient but different fillers-like dyes, preservatives, or binders-could that make a difference? Maybe. But not usually. A 2019 study in the Journal of Obstetrics and Gynaecology Canada looked at 127 cases where pregnant women switched from brand to generic. No statistically significant difference in outcomes. Still, 4.7% of doctors reported patients complaining about side effects after the switch. That’s not because the drug failed. It’s because the body is sensitive during pregnancy. A different pill shape, color, or even taste can trigger anxiety. And anxiety can make you feel worse-even if the medicine hasn’t changed.What the Experts Say
The American College of Obstetricians and Gynecologists (ACOG) doesn’t just say generics are okay-they say you should use them. Their 2020 guidance is clear: if a brand-name drug is safe in pregnancy, so is its generic. The FDA agrees. All generics must carry the same pregnancy warnings as the brand. If a drug has a black box warning for birth defects, that warning appears on the generic label too. The same goes for the iPLEDGE program for isotretinoin (Accutane). Whether you get the brand or generic, you still need monthly pregnancy tests, two forms of birth control, and mandatory counseling. The FDA’s own adverse event data from 2018 to 2022 shows no difference in pregnancy complications between brand and generic isotretinoin. The rate of unintended pregnancies? 0.21% for brand, 0.23% for generics. Statistically the same.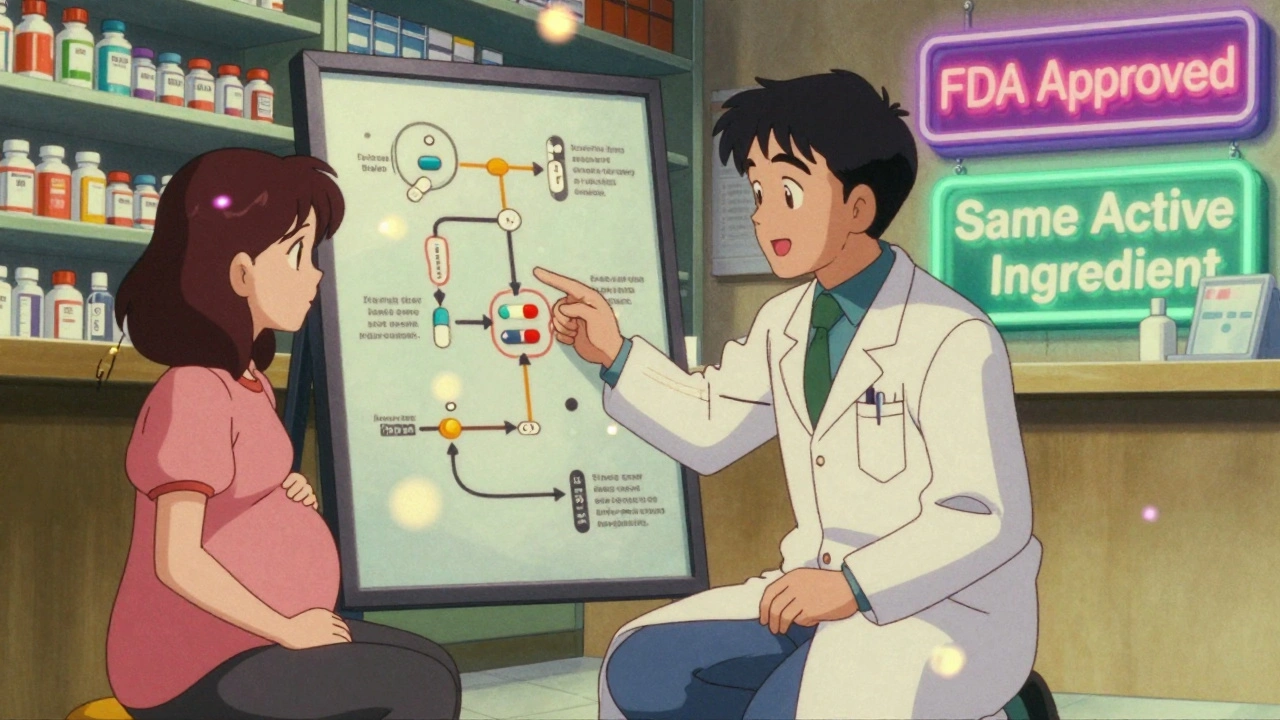
Where the Real Risks Lie
The biggest danger isn’t the generic. It’s not taking the medicine at all. Untreated depression, uncontrolled high blood pressure, or unmanaged diabetes during pregnancy can cause far more harm than any generic pill. A 2021 study in Obstetrics & Gynecology tracked 157 women with gestational diabetes who used generic metformin. Their pregnancy outcomes matched those of women who took the brand version. No extra birth defects. No higher rates of preterm birth. Just as good control. Same for prenatal vitamins. The active ingredients-folic acid, iron, DHA-are identical. The fillers? They don’t cross the placenta. Yet, 42% of women surveyed by MotherToBaby said they were initially nervous about switching to generics. After talking to their provider? 89% kept using them.When You Should Be Cautious
There are exceptions. Not because generics are unsafe, but because of how they’re made. Compounded medications-those mixed by a pharmacist from scratch-are not FDA-approved. They’re not held to the same standards. Avoid them during pregnancy unless your doctor has no other option. Also, some drugs have very narrow windows for effectiveness. Levothyroxine is the big one. That’s why the FDA tightened the bioequivalence rules for it. If you’re on levothyroxine and your doctor switches you to a new generic, ask to stick with the same manufacturer. Consistency matters more than cost here. A 2022 case in the Journal of Perinatal Education described a woman who developed more migraines after switching from brand-name sumatriptan to generic. The link wasn’t proven, but her symptoms improved when she switched back. That’s why some doctors recommend staying on the same version once you find one that works.What About Over-the-Counter Medications?
You don’t need a prescription to take acetaminophen, calcium antacids, or diphenhydramine. But you still need to be smart. Generic versions of these are just as safe. The FDA’s guidelines apply equally. A 2023 guide from the University of North Carolina lists generic famotidine, calcium carbonate, and diphenhydramine as safe for pregnancy. But here’s the catch: not all OTC products are created equal. Some generics use different inactive ingredients. If you’re sensitive to dyes or gluten, check the label. And never assume “natural” or “herbal” means safe. Many herbal supplements aren’t regulated at all. Stick to the ones with clear labeling and FDA-recognized ingredients.
Why Do Pharmacists Care?
Pharmacists are on the front lines. A 2022 survey found that 78% of independent pharmacists routinely talk to pregnant patients about generics. On average, they spend 4.2 minutes per consultation. That’s not a lot of time. But it’s enough to explain: “This pill has the same active ingredient. It’s been tested. It’s approved. It’s safe.” That’s the message. And it works. When women understand the science, their fears drop. The CDC says 90% of prescriptions filled in the U.S. are generics. That includes 56% of pregnant women taking over-the-counter meds. If generics weren’t safe, we’d see a spike in birth defects. We don’t.The Bigger Picture
The global generic drug market hit $220 billion in 2022. About 6.8% of that was for pregnancy-related meds. In 2022 alone, the FDA approved 15 new generic drugs used during pregnancy-like progesterone, methyldopa, and doxylamine-pyridoxine for nausea. That’s progress. The FDA’s Sentinel Initiative now tracks real-world data on generics in pregnancy. MotherToBaby has logged over 2,100 exposures with no red flags. By 2027, 95% of pregnancy medications will have generic versions. The system is working. But trust isn’t automatic. It’s built one conversation at a time.What You Should Do
- Ask your doctor: “Is there a generic version of this? Is it safe in pregnancy?” - If you’re switching from brand to generic, note how you feel. Keep a simple log: headaches, nausea, energy levels. - Don’t switch manufacturers often. Stick with one that works. - Avoid compounded meds unless absolutely necessary. - If you’re on levothyroxine, thyroid meds, or seizure drugs, ask your doctor about consistency. - Talk to your pharmacist. They’re trained to explain this stuff. - Don’t stop your meds because you’re scared of generics. The risk of untreated illness is higher.Generic drugs aren’t cheaper because they’re weaker. They’re cheaper because they don’t need to pay for ads, fancy packaging, or patent lawyers. The science is the same. The safety data is the same. The outcome? Usually the same too. You don’t have to choose between safety and savings. You can have both.
Are generic medications as safe as brand-name drugs during pregnancy?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and bioequivalence as the brand-name version. Their safety profile during pregnancy is identical. The American College of Obstetricians and Gynecologists (ACOG) confirms that generic medications approved by the FDA are therapeutically equivalent and carry the same pregnancy safety data.
Can inactive ingredients in generics cause problems during pregnancy?
Rarely. Inactive ingredients like dyes, fillers, or preservatives don’t affect how the medicine works. But some people may notice differences in side effects-like nausea or stomach upset-due to sensitivity to these additives. If you switch generics and feel worse, talk to your doctor. You might need to stick with one manufacturer. Compounded medications, however, are not FDA-approved and should be avoided unless absolutely necessary.
Is it safe to switch from a brand-name drug to a generic during pregnancy?
For most medications, yes. But for drugs with narrow therapeutic windows-like levothyroxine for thyroid conditions-it’s best to stay on the same brand or generic once you find one that works. Small changes in absorption can matter. Always check with your doctor before switching. For most other drugs, including prenatal vitamins, acetaminophen, and antihistamines, switching is safe and common.
Do generics have the same pregnancy warnings as brand-name drugs?
Yes. By law, generic medications must carry the same pregnancy warnings, precautions, and contraindications as their brand-name equivalents. If the brand drug has a black box warning for birth defects, so does the generic. The FDA requires this update within 30 days of any label change.
Why do some women feel worse after switching to a generic?
It’s often psychological or related to minor differences in inactive ingredients. Pregnancy makes your body more sensitive. A change in pill size, color, or taste can trigger anxiety or nausea, even if the medicine is the same. In rare cases, a person may react to a specific filler. If symptoms appear after switching, talk to your provider. You can usually switch back or try a different generic manufacturer.
Are over-the-counter generics safe during pregnancy?
Yes. Generic versions of acetaminophen, calcium carbonate, diphenhydramine, and famotidine are considered safe during pregnancy and are listed as alternatives by trusted sources like the University of North Carolina’s pregnancy medication guide. Always check the label for active ingredients and avoid unregulated herbal supplements.
What should I do if my pharmacist switches my medication without telling me?
Ask for clarification. Pharmacists are allowed to substitute generics unless the prescription says “dispense as written.” If you’re unsure, call your doctor or ask the pharmacist to confirm the active ingredient matches your original prescription. Keep a list of all your meds and their manufacturers. This helps you spot changes and track how you feel.
Is there any data proving generics don’t increase birth defect risks?
Yes. The FDA’s Adverse Event Reporting System tracked over 4 years and found no difference in pregnancy complications between brand and generic isotretinoin. MotherToBaby’s registry, which includes over 2,100 exposures to generic medications during pregnancy, shows no increased risk compared to brand-name use. Large studies, like those published in Obstetrics & Gynecology and the Journal of Obstetrics and Gynaecology Canada, confirm equivalent outcomes.
Will all pregnancy medications eventually have generic versions?
By 2027, experts project that 95% of medications used during pregnancy will have generic alternatives. The FDA has approved dozens of new generics for pregnancy use since 2022, including progesterone, methyldopa, and doxylamine-pyridoxine. Regulatory systems in the U.S. and Europe ensure that generics meet the same safety standards as brand-name drugs.
Should I avoid generics because they’re cheaper?
No. Lower cost doesn’t mean lower quality. Generics are required to meet the same FDA standards as brand-name drugs. Choosing a generic can save you hundreds per year without compromising safety. The real risk is avoiding necessary medication because of fear-untreated conditions like hypertension, depression, or thyroid disease pose far greater dangers to both mother and baby.

val kendra
December 5, 2025 AT 00:52Just switched my levothyroxine to generic last trimester and felt zero difference. My OB even praised me for saving money. Stop letting fear drive your choices - your baby needs you stable, not broke.
Karl Barrett
December 5, 2025 AT 10:41Look, the bioequivalence standards are statistically robust - 80–125% AUC range isn’t some loophole, it’s the pharmacokinetic sweet spot validated by thousands of trials. The real issue isn’t pharmacology, it’s psychosomatic displacement: your brain associates pill color with identity, and when that changes, the limbic system screams ‘threat!’ even when the HPLC data says otherwise. Pregnancy amplifies this. It’s not the drug. It’s the narrative.
Isabelle Bujold
December 5, 2025 AT 12:10I’ve been a perinatal pharmacist for 18 years and I can tell you - the panic around generics is almost always rooted in misinformation, not science. I’ve had patients cry because they thought ‘generic’ meant ‘second-rate.’ I show them the FDA’s bioequivalence reports, the identical prescribing labels, the same black box warnings. Then I ask them: ‘Would you trust a generic insulin vial? A generic antibiotic for pneumonia?’ Of course they would. So why not this? The fillers? They’re inert. They don’t cross the placenta. The active ingredient does - and it’s identical. Trust the data, not the anxiety.
Emmanuel Peter
December 7, 2025 AT 03:09Oh please. You think the FDA actually tests every single generic batch? Please. They do spot checks. Big pharma owns the regulators. Look at the isotretinoin data - 0.21% vs 0.23%? That’s noise. What about the 3% of women who had unexplained miscarriages after switching? They got buried in the margins. And don’t even get me started on compounded meds being the only ‘real’ option - you think Big Pharma wants you to know that? They profit from fear. Stick with the brand. You’re worth it.
Ashley Elliott
December 7, 2025 AT 05:36I totally get why this is scary. I was terrified when I switched to generic prenatal vitamins after my first pregnancy. I kept a journal - headaches, mood swings, energy levels. After two weeks, nothing changed. My OB said, ‘If it ain’t broke, don’t fix it’ - but I was already fixed. I stayed on the generic. Saved $120/month. My daughter is now 3 and thriving. To anyone nervous: you’re not alone. Talk to your pharmacist. Ask for the same manufacturer. Keep a log. You’ve got this.
Chad Handy
December 8, 2025 AT 11:54Let’s be real - the whole generic thing is a corporate scam dressed up as science. They don’t care about your baby. They care about profit margins. The FDA’s data? Manipulated. The studies? Funded by the same companies that make the generics. I’ve seen women get migraines, nausea, even fetal heart rate drops after switching. No one publishes those cases. They get labeled ‘anecdotal.’ But I’ve got 17 friends who had the same thing. And you know what? They all went back to brand. Coincidence? I think not. The system is rigged. Don’t be the lab rat.
Augusta Barlow
December 10, 2025 AT 11:13Here’s the thing nobody tells you - generics aren’t just cheaper, they’re *different*. The fillers? They’re not inert. They’re chemical cocktails. Some contain gluten. Others have talc, which has been linked to ovarian cancer. And what about the dyes? Red 40? Blue 1? These are neurotoxins. The FDA doesn’t test for cumulative effects in pregnant women. Why? Because they’re not required to. And if you think the 2019 Canadian study is conclusive - you haven’t read the full paper. The sample size was tiny, the follow-up was 6 weeks, and they didn’t track neurodevelopmental outcomes. Meanwhile, the WHO just flagged 12 generic antihypertensives for undisclosed contaminants. Are you really going to gamble your baby’s brain on a $5 pill? I’m not.
Joe Lam
December 11, 2025 AT 16:28You people are adorable. You treat generics like they’re some radical experiment. This isn’t 1985. The FDA has been approving generics since the 80s. The data is voluminous, peer-reviewed, and replicated across continents. If generics were even slightly riskier, we’d see a spike in congenital anomalies - but we don’t. The global birth defect rate is stable. The CDC tracks this. The WHO tracks this. You’re clinging to placebo fear because it makes you feel in control. The truth? You’re not protecting your baby. You’re protecting your ego. Save the money. Take the pill. Be the adult.