Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you want to know it does the same job as the brand-name version. That’s where bioequivalence, the scientific standard that proves two drug formulations deliver the same amount of active ingredient at the same rate in the body. Also known as therapeutic equivalence, it’s the reason your pharmacist can swap out a $200 brand drug for a $10 generic without your doctor needing to rewrite the prescription. It’s not just about cost—it’s about safety, consistency, and trust in every pill you take.
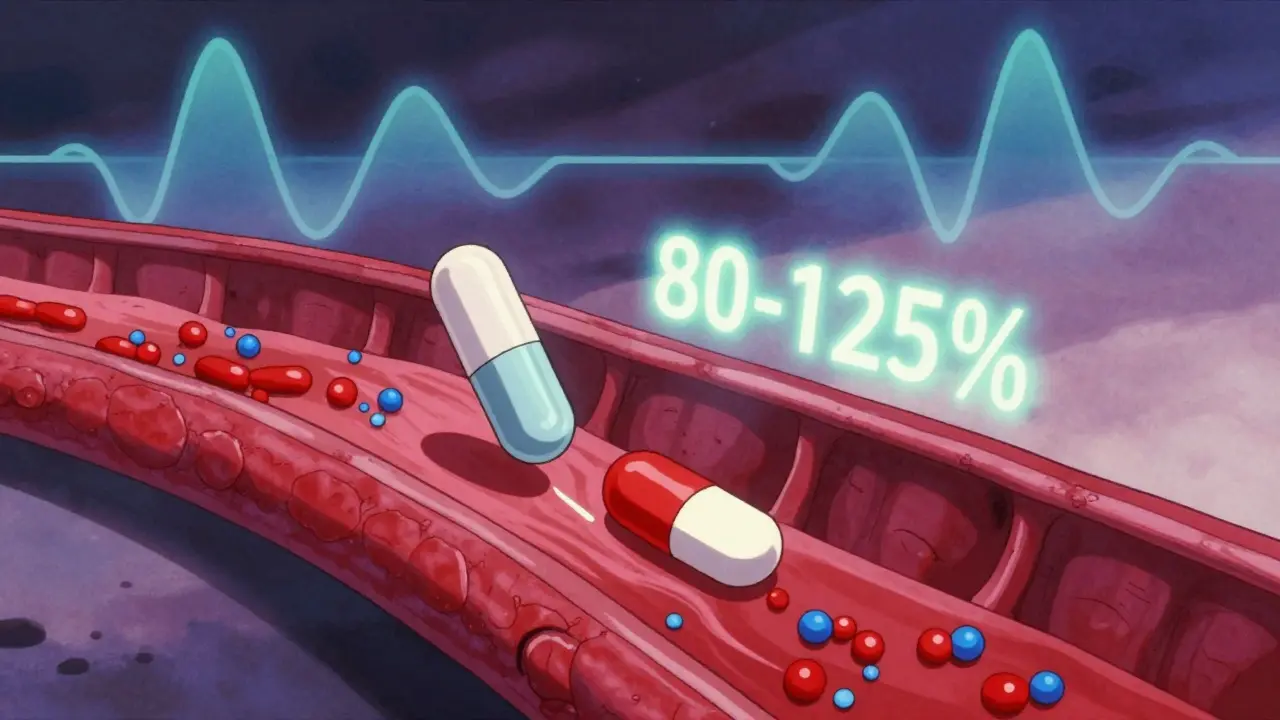
Bioequivalence isn’t a guess. It’s tested in clinical studies where healthy volunteers take both the brand and generic versions, and their blood is checked over time to see how fast and how much of the drug enters their system. If the results fall within strict limits—usually 80% to 125% of the brand’s performance—the generic is approved. This isn’t just a U.S. rule; it’s followed by the FDA, EMA, and WHO worldwide. You don’t need to worry that your generic blood pressure pill or antidepressant is weaker. If it’s labeled bioequivalent, it’s built to perform the same way.
But bioequivalence doesn’t mean identical in every way. Generic drugs can have different fillers, colors, or shapes. That’s why some people notice a change in how a pill tastes or how it dissolves. But those differences don’t affect how the medicine works in your body. What matters is the active ingredient—its amount, how quickly it’s absorbed, and how long it stays effective. That’s what bioequivalence measures. It’s the invisible guarantee behind every affordable prescription you fill.
Some drugs are trickier. Thin films, extended-release tablets, or injectables need extra testing because how they release the drug matters more. For example, a generic version of a slow-release opioid or epilepsy med must match the original’s timing exactly—or it could cause breakthrough pain or seizures. That’s why not all generics are created equal on paper, even if they’re labeled bioequivalent. The system works, but it’s not perfect. That’s why you’ll find posts here comparing generic versions of drugs like Fosamax, metformin, and sildenafil—because real people notice subtle differences, and it’s worth digging into.
When you see a drug like roflumilast, atazanavir, or ibandronate sodium listed in our collection, you’ll find posts that help you understand not just how they work, but how their generic versions stack up. Some people worry switching to a cheaper version might cause side effects or make their condition worse. Bioequivalence is the science that says it shouldn’t. But real-world experience? That’s where the stories come in. We’ve gathered posts that talk about what happens when you switch from brand to generic, how storage affects stability, and why some people still feel different on a generic—even if the numbers say they shouldn’t.
Whether you’re managing osteoporosis, diabetes, depression, or high blood pressure, knowing what bioequivalence means helps you ask better questions. It lets you save money without second-guessing your treatment. And if you’ve ever wondered why one generic works better than another, or why your doctor insists on the brand—this collection has the answers. You’ll find real comparisons, user experiences, and practical advice on how to make sure your generic meds do what they’re supposed to—without surprises.